Research in the DeLuca Lab
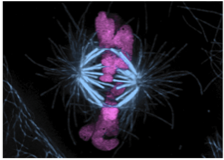
1. Generation and regulation of kinetochore-microtubule attachments.
-How does the NDC80 complex physically connect kinetochores and microtubules and how does it coordinate with other kinetochore-associated, microtubule-binding proteins to ensure formation of stable, regulatable attachments to spindle microtubules?
-How does differential Ndc80/Hec1 tail domain phosphorylation impact kinetochore functions?
-How is Aurora B kinase recruited specifically to the kinetochore to regulate attachments to microtubules during mitotic progression?
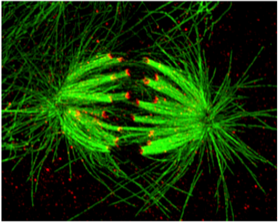
-How is kinetochore-microtubule attachment status signaled to the spindle assembly checkpoint?
-What changes occur in the protein architecture of the vertebrate kinetochore upon microtubule attachment to signal for checkpoint silencing?
-What are the molecular signals that activate Dynein to evict checkpoint proteins from kinetochores upon microtubule attachment? (In collaboration with the Markus Lab)
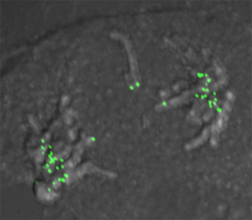
3. Kinetochore de-regulation in cancer
-How does oncogenic stress induce kinetochore defects?
-What kinetochore protein activities/functions become essential for survival in transformed cells?
-Can we exploit mitotic and kinetochore vulnerabilities in cancer cells to develop new, highly-specific therapeutics?
-in vitro biochemical reconstitution of kinetochore and checkpoint complexes
-gene editing and traditional silence/rescue assays in cultured cells
-quantitative fluorescence microscopy assays including FRAP (fluorescence recovery after photobleaching), FRET (fluorescence resonance energy transfer), BiFC (bimolecular fluorescence complementation), and PA (photoactivation)
-single molecule dynein motility assays (in collaboration with the Markus Lab)
-live-cell time-lapse microscopy: spinning disc confocal; wide-field; light sheet -super-resolution light microscopy (STORM; ExM; SHREC)
-new immunological tools to track kinetochore protein modifications in real time
